City of Hope offers clinical trials as part of our commitment to bringing our patients new and innovative cancer treatment options, especially for patients with advanced cancer or who may have run out of standard-of-care approaches. The research team at City of Hope carefully identifies and studies new and emerging treatment options that are supported by good clinical practices and scientific and investigational research. Our goal is to find those with the strongest likelihood of positively impacting our oncology patients, so we are better able to offer new treatments options.
What is a clinical trial?
Click here for an at-a-glance look at a clinical trial
Clinical studies evaluate the safety and effectiveness of new approaches to treating cancer, either with new drug development, new technologies or by using existing treatments in new ways. New treatments often take years of study before they win regulatory approval from the Food and Drug Administration (FDA). While the trials take their course, they often offer participating patients new options that may otherwise be unavailable to them, especially after exhausting conventional cancer treatments. Our research team makes one of our medical centers a clinical trial site only after careful consideration of the relative benefit to our patients.
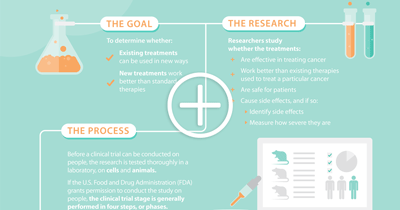
Clinical trials are an essential testing ground for studying drugs, health care products, medical devices and other treatments before they can be granted government approval. Trials with human subjects are categorized in phases (Phase 0, Phase I, Phase II, Phase III, Phase IV), with each phase bringing the treatment closer to satisfying regulatory requirements. Generally speaking, researchers study whether the treatments:
- Are effective in treating cancer
- Work better than existing therapies used to treat a particular cancer
- Are safe for patients
- Cause serious side effects
Some of these trials may study new chemotherapy drugs, surgical approaches or radiation therapy technologies. Others may explore whether a new or existing treatment works better in combination with others. One of the most exciting areas of cancer research focuses on precision treatment options. The new generation of precision drugs is generally designed to deliver more targeted, less toxic therapies. Typically, today’s precision-focused trials study new drugs that are not yet on the market, or new uses for existing drugs—to determine whether they can be used to treat additional cancer types, for example.
How do clinical trials work?
Clinical trials are tests. That may sound scary—these treatments and procedures are unproven. However, researchers must follow rigorous procedures to ensure safety, and the federal government enforces these rules. Below are key steps involved before and during clinical trials.
- An Institutional Review Board (IRB) consisting of doctors, scientists and laypeople (like you) must approve every clinical trial in the United States and make sure study participants don’t undergo unnecessary risks.
- The Data and Safety Monitoring Committee also supervises many clinical trials. Members of this committee include experts on specific conditions being studied, and they monitor the progress of trials. If they find that an experimental treatment is hurting participants, they stop the trial immediately.
- The patient must give informed consent to participate. Informed consent means researchers must communicate the reason for the study, possible benefits, the study’s design, other treatments that may be tried instead, what to expect and any known risks. Patients have the opportunity to ask questions. This part of the process keeps patients in the loop and allows them to weigh the trial’s pros and cons before committing.
- Participants have the right to leave at any time for any reason.
If you choose to participate in a study, you must sign a consent form. Next, you may expect that:
- You’ll answer questions. Researchers may ask about your medical history and likely conduct a physical examination.
- You’ll undergo standard tests. Researchers may order blood work and imaging testing, such as a magnetic resonance imaging (MRI) scan, before you start the treatment.
- You’ll have follow-up appointments and tests. You may continue to meet with your care team and undergo more tests to track progress. Your trial care team may want to know whether you’re experiencing side effects (or adverse events), including any unusual symptoms. Since clinical trials test new approaches, they may not know all of the potential side effects. Your answers may help them learn more about the treatment and protect you.
Do you qualify?
Because every clinical research study is different, the eligibility criteria vary from trial to trial. Trial qualifications may limit enrollment to those with a particular type or subtype of cancer or a specific genomic mutation, or a particular age range or cancer stage. Many trials enroll participants who have exhausted conventional treatment options or who are no longer responding to those treatments. Not every patient is a candidate for available clinical trials. Your City of Hope care team will work with you to determine if you qualify for an existing trial and, if so, will assist you in enrolling.
Search our clinical trials
How to determine whether a clinical trial is right for you
People have various reasons for participating in clinical trials. It’s a common misconception that the only people who choose to join one are doing so because they don’t have other treatment options. That may be the case for some, but other benefits to participating in a clinical trial include:
- Receiving a new treatment for a disease before it’s widely available
- More frequent monitoring of your health by researchers and doctors
- Helping to improve treatments for others in the future. For example, a breast cancer patient who knows the disease is hereditary may want to participate in a clinical trial to help future family members.
Though there are benefits to clinical trials, it’s important to consider possible risks, including:
- There may be serious side effects, and treatment may be difficult to tolerate.
- There’s a chance the treatment won’t work, or that standard treatment is better.
- You may not get the treatment. Clinical trials typically involve a control group. If you’re in that group, you’ll either get the standard treatment or a placebo. However, cancer trials rarely involve a placebo, and researchers must inform you if the study uses a placebo.
- The more frequent check-ups may be inconvenient or require long-distance travel.
Questions to ask
Before joining a clinical trial, consider asking the following questions:
- What is the purpose of this clinical trial?
- Am I a good candidate for this clinical trial?
- Why do you think this treatment is better than what’s currently available?
- What are some reasons it may not be better?
- What are the risks or potential side effects, and how do they compare with standard treatments?
- How would you know whether the treatment is working?
- Will I have to pay for the treatment, or will my insurance cover any of the costs?
- Who pays if I’m injured during the trial?
- How may this trial affect my daily life?
- How long is the trial?
- Who will be in charge of my care during the trial and communicate with me if I’m having issues such as side effects?
- Where will my appointments take place?
- When do you need to know whether or not I want to participate?
- What happens if I leave the trial early?
How to prepare for a clinical trial
Before participating in a clinical trial, consider these steps:
- Have details ready. Have the information on your cancer, such as type and stage, and your medical history ready to discuss with researchers. Compile a list of medications you’re currently taking and the dosage information.
- Call the insurance company. Your plan may help offset costs.
- Get directions. The trial may not take place at your local hospital or doctor, so be sure to ask for the address and directions.
- Bring a pen and paper. It may be hard to remember all of the important information after an appointment. Have a place to write down answers to your questions, important instructions, and the name of your point of contact.


